
Greetings iMedDo Newsletter Subscribers! Hope you are all having a wonderful summer. For this month’s episode, we meditate on Manganese! Manganese is a trace mineral nutrient surrounded in much mystery. It appears to be very evolutionarily ancient involved in energy metabolism and oxygen usage, where manganese interacts with many pathways, including calcium, magnesium, copper, and zinc and surprise! Iodine and thyroid hormones! Part one is for my general audience, and part two gets heavy into manganese biochemistry for my advanced readers. If you read this article you will be well on your way to Mastering the Manganese Mystery!
MANGANESE ON PERIODIC TABLE
Manganese (Mn) is element #25. It’s a transition metal in period 4 (4th row), group 7 (column 7).
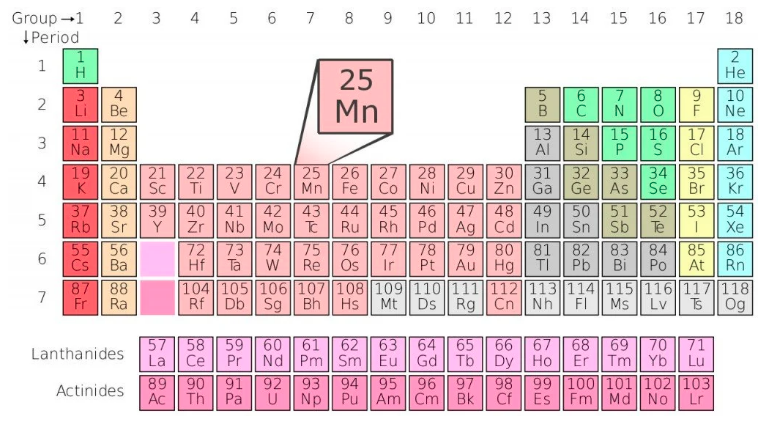
Manganese #25 is flanked by Chromium (Cr) #24 and Iron (Fe) #26, and it’s in the same column (Group 7) as Technetium (Tc) #43, Rhenium (Rn) #75, Bohrium (Bh) #107. Don’t confuse Manganese (Mn) with similarly sounding element Magnesium (Mg) which is #12.
MANGANESE FROM MAGNESIA
The words Manganese and Magnesium sound very similar because they are both from the same root word Magnesia an area of Greece & Turkey settled by the Magnetes tribe previous newsletter on Magnesium for more discussion. There was both a black ore and a white ore from Magnesia, and it is the black one that contains Manganese [the white ore contains Magnesium]. The manganese containing black ore from Magnesia is now know by the name pyrolusite which means “fire wash” and was used in ancient glass making to wash color out of glass as well as had applications for making different colors of stained glass.

Not only can manganese be used to remove color, it can be found & used in a variety of colors including even green, red, and purple. My favorite is the pink/red mineral composed of Manganese carbonate called Rhodocrosite.

Gem quality pieces of Rhodocrosite can look even more beautiful than a ruby! The most famous specimen is the “Alma Queen” on display at the Houston Museum of Natural Science which is simply amazing here’s a picture! Wow!

Rhodocrosite (Manganese Carbonate) Gem the “Alma Queen”
MANGANESE SELF LOVE
Last month I talked about the Ben-Ben stone on top of pyramids in episode Happy Slave Mind Control and told you that the secret to resisting the mind control is to love yourself more. Well it turns out that Metaphysically the Manganese gemstone Rhodocrosite if for Self-Love so that’s just perfect!
CHEMICAL CHAMELEON: POTASSIUM PERMANGANATE
In 1659, Swedish chemist Glauber fused pyrolusite ore (manganese dioxide) and potassium carbonate to obtain a material that when dissolved in water was GREEN (potassium manganate), which slowly color shifted to VIOLET (potassium permanganate), and then finally to RED a reaction now known as the “chemical chameleon” which was the first production of the super cool and useful PURPLE manganese containing chemical known as potassium permanganate (KMnO4).

Potassium permanganate (KMnO4) in liquid form is a strong oxidizing agent; and thus has many uses and is even a medicine in its own right comparable to hydrogen peroxide but stronger. Permanganate ions can be used as a germicidal antiseptic like iodine, and for water purification, but like hydrogen peroxide, requires dilution and careful dosing or can be dangerous if you get it in your eyes or ingest. I always remember potassium permanganate because I got to play with it and extract beautiful purple crystals in organic chemistry lab at Texas A&M University circa 2002. You can also combine potassium permanganate and glycerin as a firestarter as a cool survival trick.

MANGANESE METAL
Manganese when in pure metallic form looks silvery in color and forms natural cubic crystal structure. When manganese metal flakes oxidize, they can turn a variety of colors. Below is a picture of manganese in metallic cube form as well as chips of manganese oxidized which you can see can also be a variety of colors (black, brown, blue, greenish etc).

MANGANESE INDUSTRIAL USES
The most common usage of manganese is as an alloy with iron to make steel. Manganese like Magnesium can also be alloyed with aluminum and 1% of aluminum cans are made of manganese. Also a large amount of manganese is used in battery industry as manganese dioxide batteries (zinc-manganese, lithium-manganese) such as common flashlight batteries. Manganese is also used in a fuel additive called methylcyclopentadienyl manganese tricarbonyl (MMT), and in a fungicide pesticide called Maneb. It is possible for people who work in mining, smelting, welding, or battery industries breathing in manganese fumes, or exposed to high manganese containing water, to get exposed to too much manganese which can cause a Parkinson’s like neurodegenerative problem called Manganism.
MANGANESE CAVE PAINTINGS
Manganese mineral may have had more of an impact on humans than you might have realized. It turns out that the brown and black cave paintings such as the ones in Lascaux, France from 30k BC were made from Manganese oxide.

NEANDERTHAL MANGANESE NUTRITION
Manganite powder has been a common find in Neanderthal archeology sites. It is believed that the Neanderthals making cave paintings with manganese oxide were also using it as fire-starter! We will see that modern humans mostly get manganese nutrition from fruits, vegetables, nuts and grains, but even if neanderthals mostly ate only meat, it is unlikely they would be manganese nutrient deficient since they touching manganese ore for their art & smoking manganese ore in their fire-pits, an only a small amount of manganese is required for human function.
HOW MUCH MANGANESE WE NEED?
So how much manganese does a human need anyway? For quick answer let’s look at the NIH Fact Sheet for Manganese:
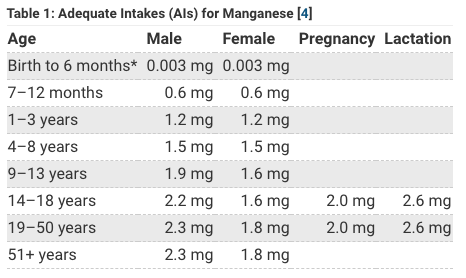
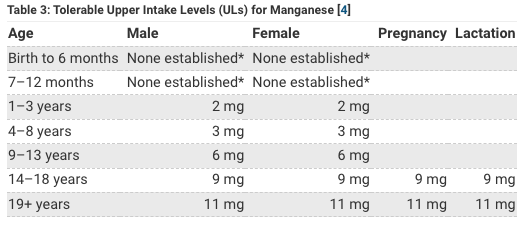
Spitballing from the above tables it looks like humans only need about 2 mg of Manganese a day and adults can get up to about about 11 mg of manganese a day with no problem. The thing that jumps out is that the government thinks women need less manganese than men in general (1.6-1.8 mg Mn for women) but (2.2-2.3 for men) yet think women need more manganese when pregnant or breastfeeding (2.0-2.6 mg).
Why do women need less manganese you might be wondering? I don’t think they do, I think this has to do with iodine deficiency & is related to iron. An important thing I learned is that both iron and manganese use the same receptors to absorb in the gut. So if you get too much manganese it could theoretically block iron absorption, and because many women are iron deficient (anemia) maybe that is why the government is recommending lower manganese for women than men. However, I think anemia for most women is being caused by either heavy metals like cadmium toxicity and/or low iodine (iodine deficiency), so instead of worrying about being low or high manganese just get more iodine (iMedDo detox system with NeuIodine) and when you iodine replenish and detox both your iron and manganese levels should return to normal.
Babies need more manganese but no-one really knows exactly how much more so the government just says that there is no upper limit established* with note that baby should just get manganese from breastmilk or formula and recommends 0.003 mg for babies 1-6 months or 0.6 mg Mn 7-12 months. Likely they are saying so low for babies because they put iron in formula and are recommending formula 1-6 months and are worried that too much manganese will block iron absorption. My position is don’t use formula use breastmilk but if you want a comparison see article Breastmilk & Multivitamins where I compare formula to breastmilk to egg to multivitamin. All 4 contain trace manganese and natural breastmilk contains approximately 0.1 mg Manganese which is higher than either cow or goat milk.
SUPPLEMENT MANGANESE OR NOT?
Because manganese is easily obtained through diet and because too much manganese is possible I do not recommend you supplement any manganese in your diet other than what you get from eating healthy and small amount in multivitamin. I take the Power Source Once multivitamin created by Dr Calin Pop which has 10 mg Manganese (gluconate) 435% DV per serving. But each serving is 8 capsules so actually each capsule has where each serving is 8 capsules; so each capsule has 1.25 mg Manganese and I usually average about 1-2 capsules a day (I take less than the recommended dosage), so I probably get 1.25-2.5 mg Manganese a day from my multivitamin not including any sources from my diet. DrPop created his multivitamin recommendations for people with genetic problems who may need more nutrients than typical recommended daily values so you might need to take more but even if you take the full dosage of 8 capsules you are getting 10 mg Manganese which is still below the 11 mg recommended upper dosage for adults 19+ according to NIH table, and no doubt you can get 2-3x their upper tolerable level without any problems so I’m not worried about any overdosing on manganese in general. The one notable exception is that welders or certain occupations might be at risk for high manganese if they are breathing it in from welding fumes. When manganese levels get too hight it behaves as a quasi-heavy metal and becomes toxic and to detox you need more iodine, silver and gold is the iMedDo way. I don’t supplement manganese in general but there is a little manganese in my multivitamin.
FOODS HIGH IN MANGANESE?
If you don’t want to supplement manganese at all you can still get enough in your diet if you eat the right foods.

The NIH Fact Sheet for Manganese has a nice table of foods and their manganese levels:

Looks like seafood, nuts, grains, fruits and vegetables basically if you eat healthy already you should be getting enough manganese. If you are all carnivore, you might want to throw some pyrolusite into your fire-pit that you grilling your meat on to make sure you getting enough manganese otherwise you might want to be sure to supplement a multivitamin.
Also check out the NIH’s short 3 page Fact Sheet for Consumers for more summarized information about Manganese nutrition.
PART TWO: MANGANESE BIOMEDICAL SCIENCE (ADVANCED)
The general public doesn’t really need to know much about manganese as they get it naturally in plants and it’s very hard for them to get too much unless they are working in some manganese industry like aluminum welding or battery industry where they might get too much from aerosolized magnesium exposure. Manganese isn’t typically tested for in blood work and it’s not something most people need to worry about supplementing. That said, there is wisdom to be found by meditating on manganese nutrition which gets very complex very fast, so for those of you who can hang with me enjoy as I discuss the parts of manganese biochemistry that jumped out at me, and I hope that you can meditate on it as well and find your own breakthroughs for your own applications.
MANGANESE & IRON
The best starting place to think about manganese is to note that it is found naturally with iron, it’s also a neighbor or iron on the periodic table, and at first pass it appears that this adjacency of manganese and iron has highly influenced life. 3 observations linking manganese and iron are:
- Same receptors in the gut absorb either manganese or iron. More iron can potentially decrease manganese absorption and more manganese can potentially decrease iron absorption.
- The mitochondria have evolved to use manganese and iron together.
- Manganese in the brain when too high goes to area of high iron (basal ganglia: substantia nigra).
So when I meditate on Manganese my first observation is: Manganese is kinda like iron, it’s next to iron on the periodic table, it’s a transition metal like iron. Iron is used for ancient oxygen chemistry. Manganese appears to be used heavily for oxygen chemistry. The same pathways in the body that deal with iron also deal with manganese. Too much manganese appears to disrupt iron pathways. Many women have low iron (anemia), and thus we should also ask do they have have manganese as well?
COPPER TO IRON-MANGANESE OXYGEN EVOLUTION
Ancient life appears to have at first used copper for oxygen chemistry and then we later evolved to use iron for oxygen chemistry. Manganese first appeared on my radar when I was meditating on copper see article Copper Containment where I made the following observations:
- Manganese is used with copper in the body.
- Iron is used with copper in the body.
- Copper electricity is harnessed by the body for oxygen metabolism.
SUPEROXIDE DISMUTASE
The observation I’m making now is that both iron and manganese like copper can also be used for oxygen metabolism. Looking at copper and oxygen metabolism via superoxide dismutase enzyme we can make a very interesting evolutionary observation:
An interesting thing to note is that in humans & yeast our anti-oxidant SOD proteins uses Copper-Zinc (Cu-Zn) pair whereas our mitochondria use an iron or manganese (Fe/Mn) compared to a bacteria which uses Nickel (Ni).
SUPEROXIDE DISMUTASE-2 USES MANGANESE
In humans we have 3 superoxide dismutase genes SOD-1, 2, 3. SOD proteins 1,3 used throughout most of the body use copper and zinc whereas SOD-2 used only in the mitochondria can use either manganese or iron at its catalytic core. SOD proteins break down harmful oxygen superoxides into less harmful (but still harmful) oxygen molecule O2 and hydrogen peroxide (H202). Thus from SOD-2 we see that manganese is used as part of system that controls oxidative stress, and more SOD expression correlates with longer cell life. Thus manganese mineral nutrient is important for mitochondrial long life. Having a manganese SOD-2 in mitochondria may mean that the body can regulate mitochondrial cell death better by keeping them alive using manganese or maybe depriving the mitochondria of manganese if it detects an dysfunctional mitochondria. Iron is used in heme in cytochrome C in mitochondria to detect H202 and to control apoptosis so I note that Manganese via SOD-2 and Iron via Cytochrome C work together as an Mn-Fe pair.
LONG LIFE
Thinking about Cu/Zn & Fe/Mn in SOD we are getting really close to the secrets of long life.
According to wiki on SOD-3: “Among black garden ants (Lasius niger), the lifespan of queens is an order of magnitude greater than of workers despite no systematic nucleotide sequence difference between them.[6] The SOD3 gene was found to be the most differentially over-expressed gene in the brains of queen vs worker ants. This finding raises the possibility that SOD3 antioxidant activity plays a key role in the striking longevity of social insect queens.[6]“
Many people have too high Cu and too low Zn, and many people more common in women have too low Fe and too high Mn. This indicates a dysregulation of SOD and likely leads to shorter life from oxidative damage. I think that the key to fixing this is to get iodine and silver and gold.
High copper can be balanced by gold, NeuGold nano-colloidal gold. It’s easy to take more zinc. But how do we fix low iron? Just taking more iron doesn’t work. Low iron can be caused by toxins such as cadmium so detoxing with iodine helps. If iron is too high can try taking more manganese. Getting more manganese into the mitochondria will help it live longer whereas silver works by shutting down dysfunctional mitochondria and iodine can both promote healthy mitochondria and shut down cancerous mitochondria. So Iodine + Manganese can work together to promote healthy mitochondria. Getting more manganese into your mitochondria by up-regulation of SOD-2 could result in longer life.
PLANTS USE MANGANESE TO MAKE OXGYEN
Let’s think about manganese from a different angle. We know it’s useful in oxygen metabolism as we discussed above but it turns out it’s critical to all life because plants need Manganese to create oxygen and without oxygen we would all die since we all exist in symbiosis with oxygen generating plants. Manganese is used in plants at the oxygen-evolving-complex in light generated photosynthesis. Looking at how it works multiple manganese atoms are used with an atom of calcium to create oxygen.
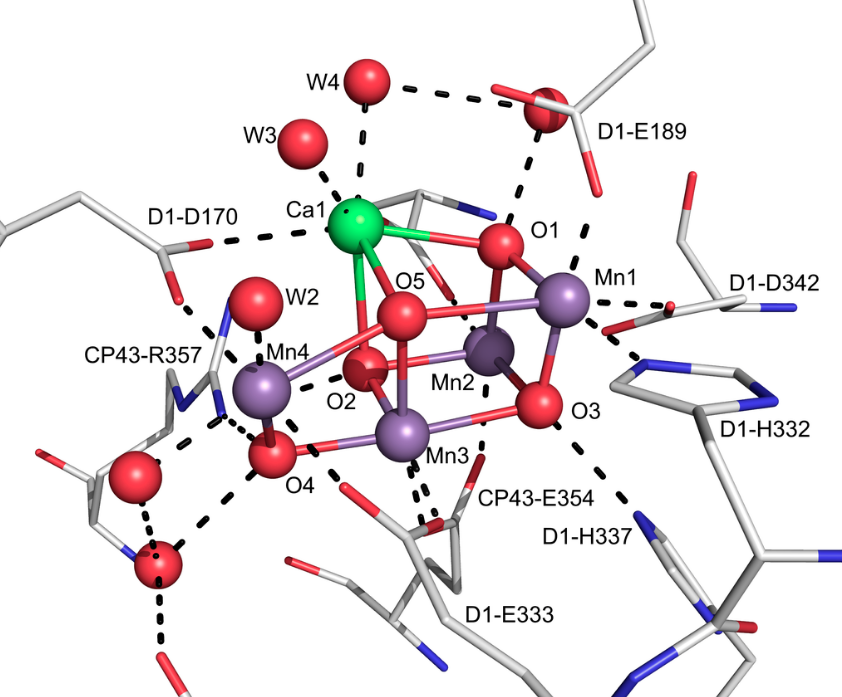
The magic happens when the fifth oxygen moelcule 05 is combined with a sixth oxygen atom (not shown) and diatomic O2 is released. Note how 05 is stabilized between two Manganese atoms (Mn1 and Mn4) as well as a calcium atom (Ca1).
So nice of plants to make oxygen for us. Even nicer that we can eat plants and get manganese nutrition because they have 4 molecules of manganese in each of their photosynthesis oxygen evolving complexes.
PLANT MANGANESE COMES WITH CALCIUM
An interesting observation is that when we get manganese naturally from eating plants, each 4 molecules of manganese we get also comes attached to a calcium atom.
So it is not surprising that manganese nutrition is somehow linked with calcium signaling. What is clear is that manganese deficiency results in bone & cartilage problems, so thinking about how calcium and bones interact with manganese is worthwhile to meditate on.
MANGANESE OVERVIEW
Manganese is used for Free Radical Defense Systems, Bone Formation, & Macronutrient metabolism. Manganese is also used in dozens of proteins and enzymes. Manganese is mostly found in the bones, also in the liver, kidneys and brain.
We already discussed how it is used in free radical defense systems via SOD-2 in the mitochondria. It makes sense that manganese is involved in bones as it comes naturally in our plant diet complexed with calcium atom. What Manganese is doing at a deeper level with the bones and metabolism is a bit of a mystery so I’ll look at the dozens of manganese proteins in more detail for clues to this manganese mystery.
MAGNESIUM & MANGANESE
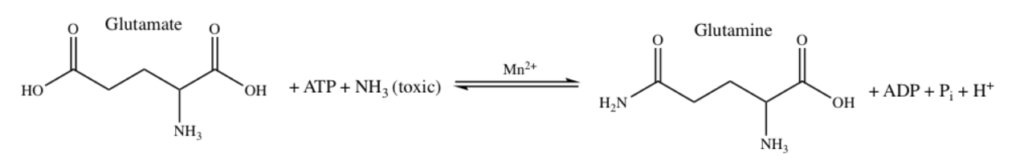
Manganese as Mn2+ form can sometimes substitute for Magnesium Mg2+ for stabilization of phosphates and thus could be used in energy pathways or enzymes that use phosphate energy (ATP, ADP, AMP etc). As an example let’s look at Manganese in detail in the brain protein glutamine synthase:
GLUTAMINE SYNTHASE
In the human brain, the manganese is bound to manganese metalloproteins, most notably glutamine synthase in astrocytes. Glutamine synthetase (GS) is an enzyme that plays and essential role in the metabolism of nitrogen by catalyzing the condensation of glutamate and ammonia to form glutamine. Glutamine as glutamic acid is an amino acid used to make proteins and also is used as a neurotransmitter. In fact glutamic acid is the most abundant excitatory neurotransmitter in the vertebrate nervous system. (Also it serves as the precursor for the synthesis of the inhibitory gamma-aminobutyric acid (GABA) in GABAergic neurons.)
Astrocytes protect neurons against excitotoxicity by taking up excess ammonia and glutamate. Because of its role in synaptic plasticity, glutamate is involved in cognitive functions such as learning and memory in the brain.
Ergo, Manganese is important for learning and memory because it is necessary in brain to temper too much glutamate or too much ammonia in the brain since manganese is necessary for glutamine synthase which converts excess glutamate and ammonia into glutamine amino acid instead.
So Manganese is part of an important brain neurotransmitter to amino acid to protein pipeline via glutamine synthesis interesting. Linking neurotransmitters to proteins to nitrogen metabolism manganese is important but what is it really doing?
I suspect Manganese as Mn2+ is being used here as an alternative to Mg2+ as a phosphate stabilizer for the ATP dependent part and of the glutamine synthase reaction which uses a phosphate intermediate. I think manganese is just helping convert Phosphate energy stored in ATP into a useful chemical reaction using the Mn as a intermediate stabilizer/co-factor.
To confirm I have to look at GS in more detail and figure out where the Manganese part is located and found this resource from a college inorganic chemistry course to help.
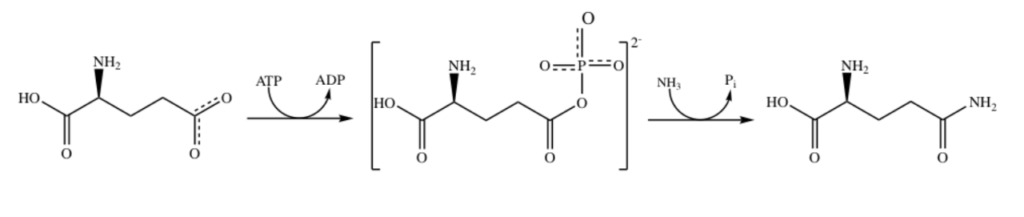
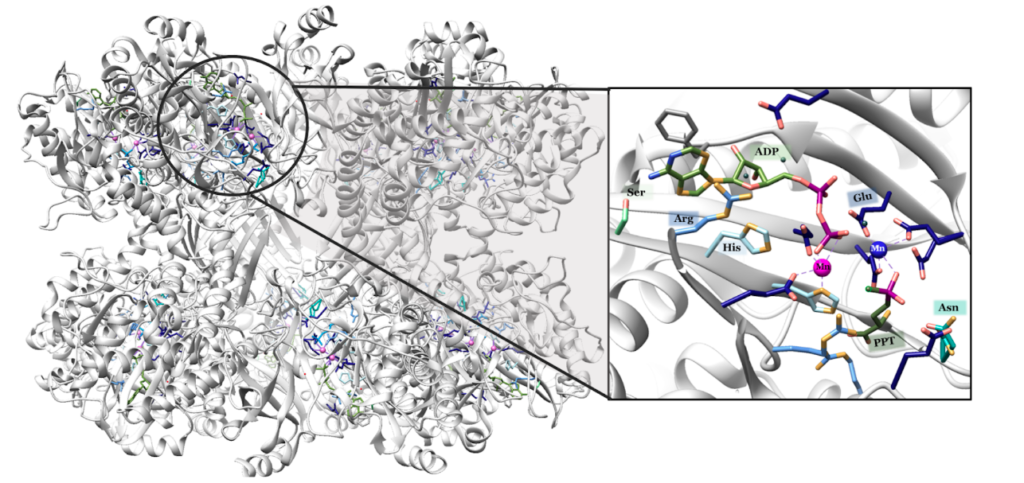
“Each active site can be described as a bifunnel in which ATP binds at the top and glutamate binds at the bottom. Two metal cations lie at the center of each active site. The enzyme is functional with either Mg2+ or Mn2+ in the active site, however Mn2+ is more common despite the fact that it slightly slows down the activity of the GS enzyme.5 GS catalysis is faster with Mg2+, however GS selects Mn2+ because it binds to the enzyme with higher affinity.7 “
Okay that was lot of work but it was worth it. Yes I confirmed that that as I suspected Manganese is Mn2+ is functioning exactly as I would expect Magnesium as Mg2+ to behave. Mg2+ stabilized Phosphates and it looks like my hypothesis that Mn2+ can do the same is correct since this GS protein can be functional with either.
For the brain timing is important so the observation that the Mn2+ does the same as Mg2+ but slightly slows down the GS enzyme is interesting especially when you read about the GS protein in detail it has many other modulators (glycine, serine etc)
Turns out in GS there are actually TWO Manganese atoms used, one as a catalyst (blue) and the other as as the phosphate stabilizer (pink)
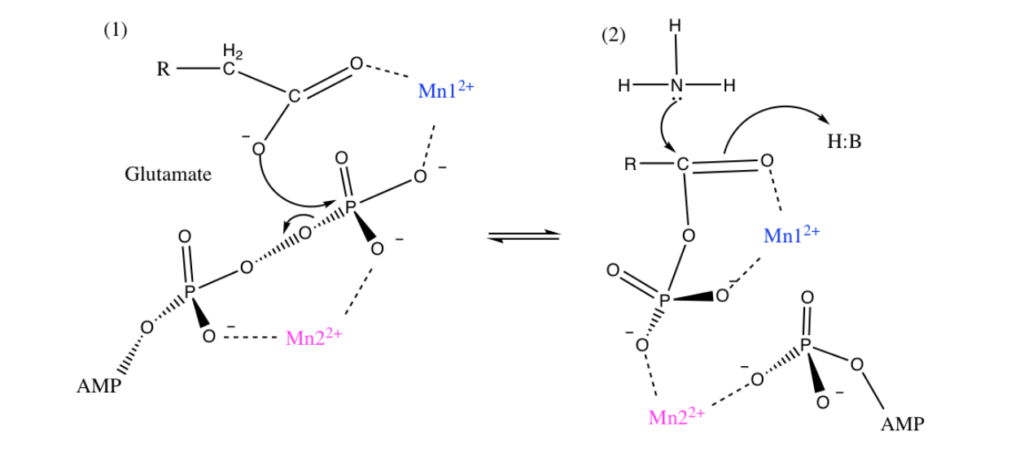
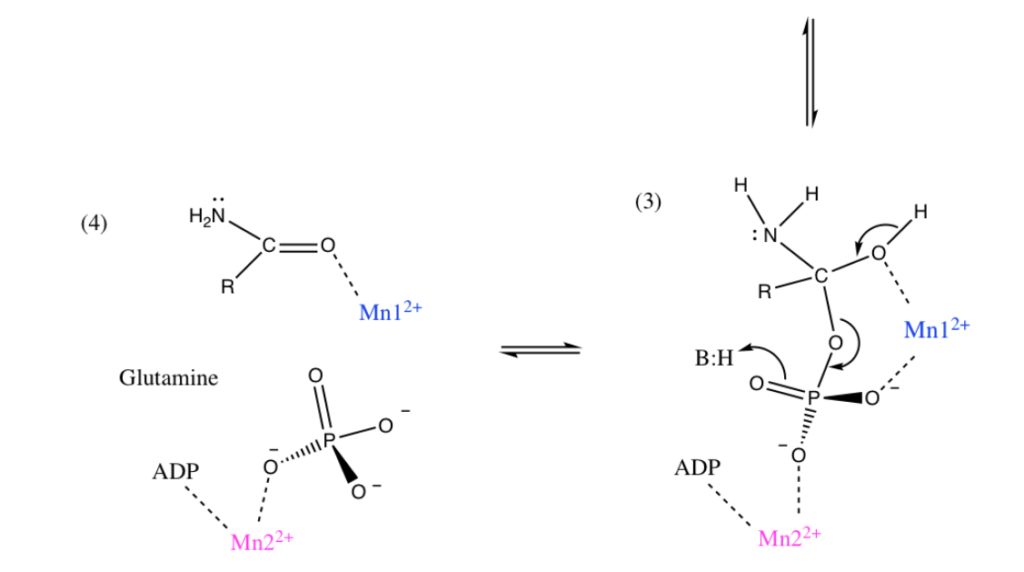
Malfunction of GS in the brain can lead to neurological diseases such as Alzheimer’s, epilepsy, glioblastoma multiforme, anxiety and depression. Manganese plays a central role in how this GS enzyme works as it needs Mn to function but can substitute with Mg if needed. It’s nice to know such an important pathway has a backup system. Also note that if your brain runs out of both Manganese and and Magnesium you are in trouble.
ENZYMES THAT CONTAIN MANGANESE
Besides SOD-2 and glutamine synthase which we talked about above, other manganese containing enyzmes include oxidoreductases, transferases, hydrolases, lyases, isomerases, & ligases.
ARGINASE
Arginase is a manganese containing enzyme of the hydrolase family, the final enzyme in the urea cycle. It is ubiquitous to all domains of life. It turns arginine plus water into ornithine plus urea. In humans and all mammals it allows the disposal of harmful ammonia into urea to be excreted via urine. Mn2+ ions coordinate with water, orienting and stabilizing the molecule and allowing water to act as a nucleophile and attack L-arginine, hydrolyzing it into ornithine and urea. Arginase-1 is located in the cytoplasm and hepatocytes (liver cells). Arginase-II is located in mitochondria and several tissues most abundantly in kidney and prostate. Arginase is expressed with nitric oxide synthase in smooth muscle, and is a controlling factor in both male erectile function and female sexual arousal. Therefore, manganese dysregulation can result in problems with sexual function.
PYRUVATE CARBOXYLASE
Pyruvate carboxylase (PC) is an enzyme that turns pyruvate into oxalate and requires either magnesium or manganese + Acetyl-CoA to function. As we saw earlier in the glutamine synthase enzyme as well, Mg2+ or Mn2+ are being interchangeable here because function is to stabilize phosphate residues during ATP dependent energy steps.
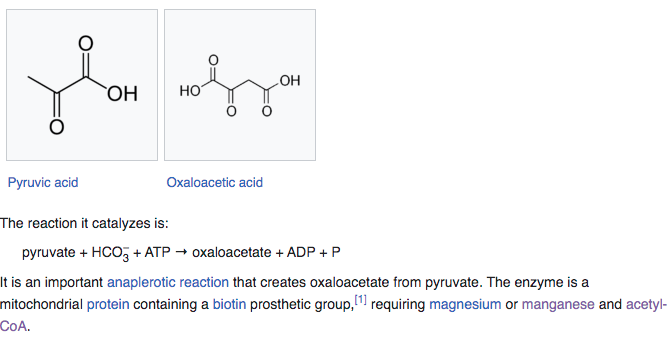
Pyruvate carboxylase uses a covalently attached biotin [Vitamin B7] cofactor which is used to catalyze the ATP– dependent carboxylation of pyruvate to oxaloacetate in two steps. Because PC enzyme is used in gluconeogenesis, the production of sugar, connections between manganese and diabetes suppression have been postulated.
When manganese is used it also requires an Acetyl-CoA a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism.
MANGANESE ABSORPTION
Manganese is absorbed in the small intestine through an active transport system, and possibly through passive diffusion as well when intake levels are high. After absorption, some manganese remains free, but most is bound to transferrin, albumin, and plasma alpha-2-macroglobulin. Manganese is taken up by the liver and other tissues, but the mechanism of this process is not well understood. Humans absorb only about 1-5% of dietary manganese and infants and children tend to absorb more. Infants absorb much more 20% of dietary manganese. Manganese absorption is regulated as evidenced by absorption efficiency increasing with low manganese intakes and decreases with higher intakes, but little is known about the mechanisms that control absorption. Men tend to absorb Mn less efficiently than women possibly because men tend to have higher iron status.
BULK MANGANESE
The human body contains about 10-20 mg manganese of which 25% -40% is in the bone. The liver, pancreas, kidney and brain also contain manganese.
*I think every cell in the entire body contains some manganese since is used in the mitochondria and all cells have mitochondria. (SOD-2 protein for example).
MANGANESE ELIMINATION
More than 90% of absorbed manganese is excreted via bile into the feces, and a small amount is reabsorbed. Very little is excreted in urine.
*Even though manganese is not excreted much in the urine, it’s effect on creating urine cannot be overstated since is used in arginase to make urea (the main component of urine). So I’m really surprised to hear not much manganese comes out of the urine.
MANGANESE & IRON
Dietary iron intakes and iron status (measured from serum ferritin concentration) appear to be inversely associated with manganese absorption. The mechanism for this effect is unknown but the shared transporter of iron and manganese in the intestine might play a role.
MANGANESE ABSORPTION BLOCKERS
Iron rich foods or supplements have been show to lower absorption of manganese. Phosphorus and calcium may also decrease your retention of manganese but at a lower amount compared to iron.
DIETARY SUPPLEMENT TYPES
Mn in dietary supplements is present in many forms including amino acid chelates (e.g. manganese glycinate chelate, bisglycinate chelate, manganese glycinate chelate, and manganese aspartate). Other forms include manganese gluconate, manganese picolinate, manganese sulfate, manganese citrate, and manganese chloride.
*The multivitamin I take and recommend uses Manganese Gluconate. PowerSourceOne uses Manganese (Gluconate) 10 mg 435% DV per serving. Serving is 8 capsules so is 1.24 mg Mn/capsule. I don’t recommend taking stand alone manganese supplements just get the small amount of manganese you need from plant diet and multivitamin is plenty. However if you have HIGH iron confirmed by blood work you might consider taking a manganese supplement but in general is unlikely you are low in manganese, and be careful as too much manganese can become toxic.
MANGANESE TOXICITY IN WELDERS AND WATER
Manganese can have neurotoxic effect in large amounts. This is uncommon unless you are inhaling manganese or are drinking water with too high manganese levels. Inhaled manganese say if you are an aluminum welder (1% manganese in aluminum alloy) can cause inflammation of the lungs with symptoms that might include cough and bronchitis. High levels of manganese (>300 microgram Mn/L) are associated with reduced intellectual function in children. Manganese is also required for proper baby brain development and is not inherently neurotoxic.
*When you are iodine deficient it’s more likely that manganese will be toxic to too you whereas when you detox your body can eliminates any excess metals it doesn’t need.
*It’s still better to have well water with too much manganese in it than it is to drink fluoridated water.
MANGANISM
“Exposure to high levels of manganese during fetal development can result in neurocognitive deficits, which in adults; manganese accumulation in the basal ganglia can lead to decreased dopamine levels and cell death in a syndrome called manganism which shares multiple features with Parkinson’s disease.” Early symptoms of manganese neurotoxicity include fatigue, headache, muscle cramps, loss of appetite, apathy, insomnia, and diminished libido. Manganism is initially characterized by the psychiatric disorder, locura manganica with symptoms including compulsive or violent behavior, emotional instability, and hallucinations.
MANGANESE FROM PLANTS
The most natural source of manganese is from plants. The reason is that plants use manganese for photosynthesis as part of their oxygen evolving complex (OEC). Manganese is used with calcium for function of oxidizing individual oxygen molecules into oxygen O2 diatomic molecules which plants release into the atmosphere. So without manganese used by plants we wouldn’t be here because we need oxygen to live.
DIGESTION
Manganese helps with protein and amino acid digestion and utilization, as well as the metabolism of cholesterol and carbohydrates.
VITAMINS
Manganese helps body utilize a number of vitamins such as choline, thiamine, and vitamins C and E, and ensures proper liver function.
BLOOD CLOTTING
Manganese plays a role in blood clotting and hemostasis in conjunction with vitamin K.
VASODILATION
Manganese is a known vasodilator, which means it helps enlarge veins to efficiently carry blood to tissues like the brain.
PANCREAS
Manganese is heavily concentrated in the pancreas. It’s involved in the production of insulin, which removes sugar from the blood. Thus, manganese may contribute to the proper secretion of insulin and help stabilize blood sugar.
THYROID
Manganese plays a role in the production of thyroxine. Thyroxine (T4) contains 4 molecules of iodine and is the inactive form of thyroid hormone. Thus there is a connection between manganese and my favorite topic iodine. Iodine deficiency and heavy metal toxicity and halogen toxicity results in defective or dysregulated thyroid hormones and other dysregulation of minerals such as manganese which is a problem if it either gets too high or too low. It’s almost impossible to run out of manganese in your body as abundantly found in plant diet and stored in your bones, but it is easy for the manganese to get too high if your body is asking for more iodine and can’t find it. Body sends the signal to make more iodine containing thyroid hormones (T4) via TSH via brain dopamine which is affected by manganese levels. Too much manganese has bad effect by accumulating in high iron part of the brain in the basal ganglia known as the substantia nigra and can disrupt dopamine as well as TSH and other thyroid hormones. Manganese deficiency could cause or contribute to hypothyroid condition, which may contribute to weight gain and hormone imbalances.
REFERENCE: EFFECTS OF MANGANESE ON THYROID HORMONE HOMEOSTASIS
*Don’t worry about being low in manganese for your thyroid just get more iodine and it should fix itself as you detox hormones and mineral levels including manganese should rebalance. It’s very hard to run out of manganese unless you just completely stop eating any plants. For carnivores doing a paleo diet maybe you need to throw some manganese into your fire-pit like the Neanderthals did if you don’t want to eat any plants. Humans are omnivores so eat some meat and plant matter is my personal strategy.
DOPAMINE
“Dopamine is a known inhibitory modulator of thyroid stimulating hormone (TSH) secretion. The involvement of dopamine and dopaminergic receptors in neurodevelopment, as well as TSH modulation led us to hypothesize that excessive manganese exposure may lead to adverse neurodevelopment outcomes due to the disruption of thyroid homeostasis via the loss of dopaminergic control of TSH regulation of thyroid hormones”
*The paper hypothesizes that too much manganese can adversely affect your brain, dopamine and thyroid hormone regulation.
OSTEOARTHRITIS
Combining manganese with glucosamine and chondroitin may reduce osteoarthritis pain.
*I recommend Frankincense and berries for osteoarthritis (NeuGold for Rhematoid arthritis).
WOUND HEALING
Manganese is present in an enzyme that provides amino acid called proline. Proline is necessary for the production fo collagen in your skin cells. Collagen formation is essential to wound healing, and wound healing can be slowed down with manganese deficiency.
*Manganese deficiency is rare, I put Neusilver on wounds for wound healing.
Some research shows that putting manganese calcium and zinc on chronic wounds for 12 weeks may improve wound healing.
*Zinc does help with some skin conditions, had a friend break out in a chlorine rash after getting into a over-chlorinated pool and she broke out in hives. I had her apply zinc oxide suncreen and it made the itching stop. Whether manganese mixed with calcium and zinc is better for wound healing than just zinc I’m not sure but worth a shot. Typically I just use NeuSilver on skin wounds and they heal very fast usually without a scar.
FERTILITY
Manganese deficiency though rare can result in low fertility such as testicular shrinkage. Manganese deficiency in animals has been found to cause skeletal defects like curved spine, shorter and thicker limbs and enlarged joints.
MENSTRUAL CYCLE
Low levels of manganese might contribute to pre-menstrual syndrome (PMS).
*I’ve heard of women taking NeuGold to help with PMS, but apparently taking manganese might help as well. I’m not a woman so ladies let me know if works. What works better for PMS: NeuGold or Manganese? If anyone has any data on or personal experience on that let me know.
From what I know now, I suspect you might also want to try taking Magnesium as both Gold is known to unblock the +2 divalent metal pathway that is blocking probably both Manganese Mn2+, and Magnesium Mg2+, and also maybe try zinc (Zn2+) and calcium (Ca2+)
DIVALENT METAL TRANSPORTER
Neurons, contain divalent metal transporter (DMT1). DMT1 is a major transporter for manganese and is functionally important with regard to the necessity of stable homeostasis of manganese in in extracellular fluids for optimal brain function. Rat studies show that injected manganese within 5 minutes can be found in the choroid plexus and then cerebrospinal fluid indicating it was let in by DMT1 transporter.
IMMUNE SYSTEM
Manganese may have a role in the immune system for natural immunity along with iron and zinc. “Unlike zinc, there is little information regarding the effects of manganese deficiency on immune development and function. There are, however, limited data suggesting that toxic levels of manganese may impair immune function. Further, emerging data have revealed that vertebrates resist bacterial infections through manganese sequestration.”
*Invading pathogens fight with your body for iron. Iron feeds bacteria. It’s not surprising they might fight for manganese as well but the data sounds too sketchy to comment further.
CONCLUSION
My take is that Manganese nutrition is not a problem for vast majority of people. What is a problem for vast majority of people is iodine deficiency. Manganese problems and iodine deficiency overlap in symptoms because of manganese’s use in dopamine/TSH/thyroid hormone pathway. If you think your manganese is out of whack try the iMedDo detox system. Studying manganese gave me insight in iron problems, magnesium problems and let me look at iodine and thyroid neuroendocrine regulation of metabolism in a whole new light. Manganese and Iron are used instead of Copper and Zinc for mitochondrial suppression of oxygen free radical in SOD-2 which is exciting as we are narrowing in on very useful information for how to live longer when we mediate on manganese nutrition, so thank you for reading and thinking about Manganese! I’m not sure if we mastered the mystery but at least we have a good starting platform which I hope you of all reading levels found useful. For those of you with advanced training you will note how much about manganese is simply unknown, so I hope this article simply peaked your interest and will inspire some future generation to look more deeply into this and related topics.
Namaste,
DrBenGo Healthwarrior
PS Manganese from Magnesia is sacred to the Ancient Great Goddess. It has the power to make babies healthy or to murder you if you take too much or get too cocky like Niobe. Manganese meditation took me to a dark but beautiful place in human history where slaves working in mines and went insane, rebelled when they learned the truth that we are all one with God and created in God’s image. Pyrolusite is a component of the Philosopher’s Stone. Great Goddess energy is back as we are all being treated like mine slaves again with the covid jabs, the results never end well. End of civilization for some, start of a new Golden Age for others.


